There are very surprising and interesting implications of proofreading for the way DNA is replicated. As summarized in the Alberts textbook:-
"The need for accuracy probably explains why DNA replication occurs only in the 5′-to-3′ direction. If there were a DNA polymerase that added deoxyribonucleoside triphosphates in the 3′-to-5′ direction, the growing 5′-chain end, rather than the incoming mononucleotide, would carry the activating triphosphate. In this case, the mistakes in polymerization could not be simply hydrolyzed away, because the bare 5′-chain end thus created would immediately terminate DNA synthesis (Figure 5-11). It is therefore much easier to correct a mismatchedbase that has just been added to the 3′ end than one that has just been added to the 5′ end of a DNA chain. Although the mechanism for DNA replication (see Figure 5-8) seems at first sight much more complex than the incorrect mechanism depicted earlier in Figure 5-7, it is much more accurate because all DNA synthesis occurs in the 5′-to-3′ direction.
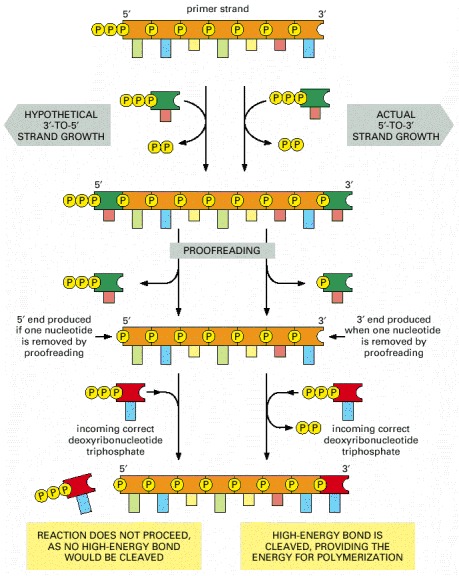
Figure 5-11An explanation for the 5′-to-3′ direction of DNA chain growth
Growth in the 5′-to-3′ direction, shown on the right, allows the chain to continue to be elongated when a mistake in polymerization has been removed by exonucleolytic proofreading (see Figure 5-9). In contrast, exonucleolytic proofreading in the hypothetical 3′-to-5′ polymerization scheme, shown on the left, would block further chain elongation. For convenience, only the primer strand of the DNA double helix is shown.
Copyright © 2002, Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter; Copyright © 1983, 1989, 1994, Bruce Alberts, Dennis Bray, Julian Lewis, Martin Raff, Keith Roberts, and James D. Watson .
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

